Description
DESCRIPTION
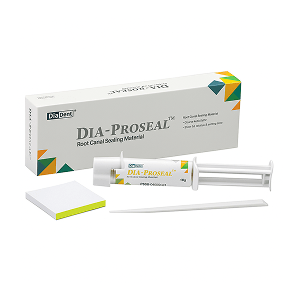
SafeEndo BIOACTIVE 3D is an advanced bioactive dental material designed to support natural tooth repair and long-term restoration stability. It promotes effective sealing and interaction with tooth structure during restorative and endodontic procedures. Suitable for professional dental use, it helps enhance clinical outcomes with reliable handling and performance. A premium SafeEndo bioactive solution for modern dental clinic practice.
FEATURES
• BioActive 3D can be used, in addition to conventional techniques, in singlecone
technique and thermoplastic filling.
• Use conventional gutta removal techniques.
Single-cone technique
1. Anesthetize, install absolute isolation and then perform the
biomechanical preparation of the canal.
2. Remove the sealing tip and attach the applicator tip to the syringe.
3. Dry the canal only with absorbent paper cones without causing
excessive dryness.
4. Apply BioActive 3D in the apical third of the canal directly with the syringe.
5. Insert the gutta percha cone.
6. X-ray for checking the correct filling of the canal.
7. Cut the cone at the desired height with heated instruments followed by vertical
compression.
8. Remove excessive material from the canal walls with suitable irrigating
solutions for this purpose, perform crown sealing and restoration.
KEY SPECIFICATION
BioActive 3D is a bioactive root canal sealer based on innovative mineralmicroaggregatechemistry “ActiveBiosilicateTechnology”thatoffers
1) Biocompatibility: high mineral purity and monomer free formulation reducing
the risk of adverse tissue reaction
2) Bioactive properties: hydroxyapatite formation at the tooth-sealer interface and
mineralization of dentinal structure
3) Alkaline pH
4) Sealing properties: crystallization of the material inside the dentin tubules
creating a tight seal
5) Retreatable : if a retreatment is needed, BioActive 3D can be easily removed
from the root canal
6) Strontium silicate base bio ceramic technology of
DIRECTION TO USE
1. Position the applicator tip in the syringe and perform the chemical
decontamination of the assembly (tip +syringe)
2. We recommend using a disposable plastic cover on the syringe to avoid
Cross-Contamination.
3. Confirm the material exists from the syringe before direct application to
thecanal.
4. Position the tip and syringe assembly properly into the canal and depress the
plunger lightly to prevent excessive output of the product.
5. Before closing the syringe, retract the plunger to stop the outflow.
6. Clean any excess material on the tip of the syringe and close with appropriate
pressure after use to prevent contact with moisture, which causes the product
to dry.
7. Clean and disinfect the syringe in subsequent uses to avoid crosscontamination; in case of contamination with saliva or blood, discard it.
Packaging
1 x 3gm syringe